Introduction:
In Multiple Myeloma (MM), nuclear exporter exportin-1 (XPO1) is a target for selinexor. Selinexor has a promising efficacy and a favorable safety profile in relapsed refractory MM. This systemic review aims to explore the efficacy and safety of selinexor based regimens for the treatment of relapsed refractory multiple myeloma (RRMM).
Materials and Methods:
We conducted a literature search using four databases (PubMed, Embase, Cochrane library, and ClinicalTrials.gov). Our search strategy included MeSH (Medical Subject Headings) terms and keywords for multiple myeloma and Selinexor including trade and generic names from the date of inception to April 13, 2020. Initial databases yielded 115 articles. After excluding review articles, duplicates, and non-relevant articles, we included six clinical trials reporting the efficacy of Selinexor in RRMM.
Results:
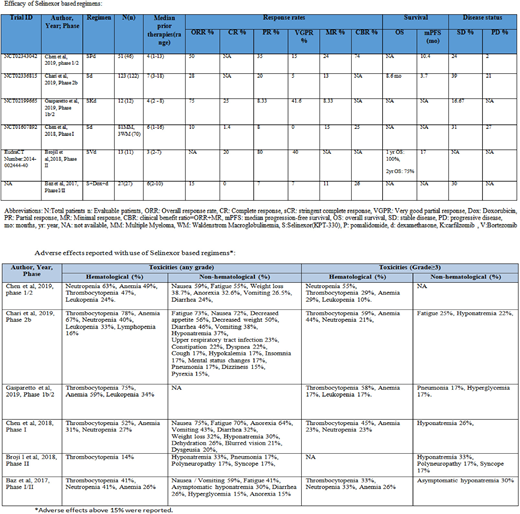
Among a total of 310 enrolled patients, 288 patients were evaluated. Selinexor was given in combination with dexamethasone to 207 and as three drug regimens to 103 RRMM patients. Chen et al. studied the efficacy of selinexor plus dexamethasone (d) in RRMM pts (n=70) in phase I trial achieved an overall response rate (ORR) of 10%, stable disease (SD) of 31% and progressive disease (PD) of 27%. Similarly, Chari et al. studied selinexor + dexamethasone in RRMM pts (n=122) in phase IIb trial, and observed ORR of 28%. The median overall survival (OS) was 8.6 months (95% CI, 6.2 to 11.3) and a median progression-free survival (PFS) was 3.7 months (95% CI, 3.0 to 5.3) with SD of 39% and PD of 21%.
Chen et al. studied selinexor in combination with pomalidomide (P) + d in RRMM patients (n=46), achieved an ORR of 50% and the median PFS of 10.4 months. The disease was stable in 24% of patients with a low rate of progression i.e. 2%. In a phase Ib/II trial using selinexor + carfilzomib + d by Gasparetto et al. (n=12), the ORR was 75%, with SD of 16.67%. Selinexor in combination with doxorubicin and d was studied in phase I/II trial by Baz et al. (n=27) with ORR of 15% and SD in 30% of patients. Brojil et al. reported the role of selinexor + bortezomib + d in RRMM (n=11) in phase II trial with partial response (PR) of 80%. The median PFS was 17 months and one-year overall survival (OS) was 100%. The majority of hematological adverse effects were pancytopenia. The main non-hematological adverse effects were pneumonia, and peripheral sensory neuropathy.
Conclusion:Selinexor showed promising outcomes in terms of ORR for the treatment of RRMM. The responses in selinexor based three-drug regimens were observed higher as compared to two-drug regimens, providing a benchmark for future studies.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal